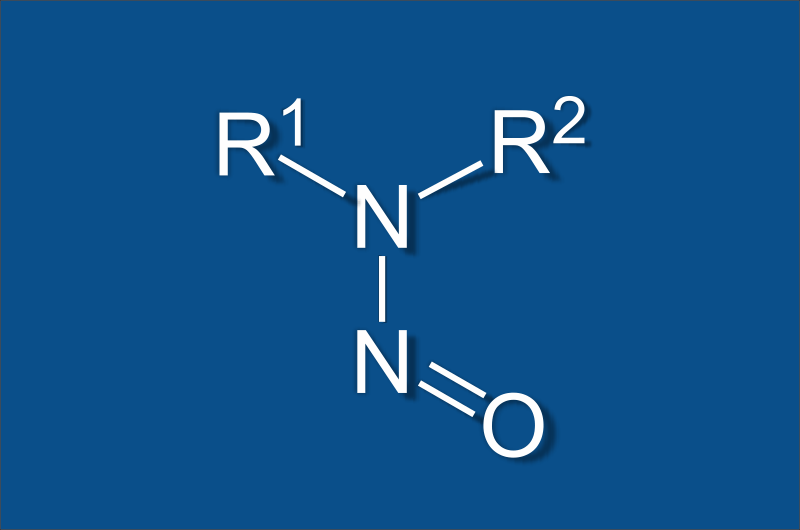
Pesticide Residue Testing with USP <561>
In order to meet the safety standards enforced by regulatory authorities, food commodities and botanicals are required to undergo pesticide residue testing. The tests described in the U.S. Pharmacopoeia <561> “Articles of Botanical Origin” and the European SANTE/11813/2017 guidance documents allow for the quantitative and qualitative analysis of these pesticide residues. These analyses are [...]