Frontage Acquires Nucro-Technics Inc. and Nucro-Technics Holdings, Inc.
August 15, 2023: Frontage Laboratories, Inc. and its wholly-owned subsidiary, Frontage Canada, Inc., completed the acquisition of Nucro-Technics Inc. and its affiliate Nucro-Technics Holdings, Inc. Nucro-Technics [...]
Nucro-Technics at the 2023 Society of Toxicology 62nd Annual Meeting and ToxExpo
As with every year in March, five thousand attendees throughout the pharmaceutical and biotech industry converge on a city in the United States for the Society [...]
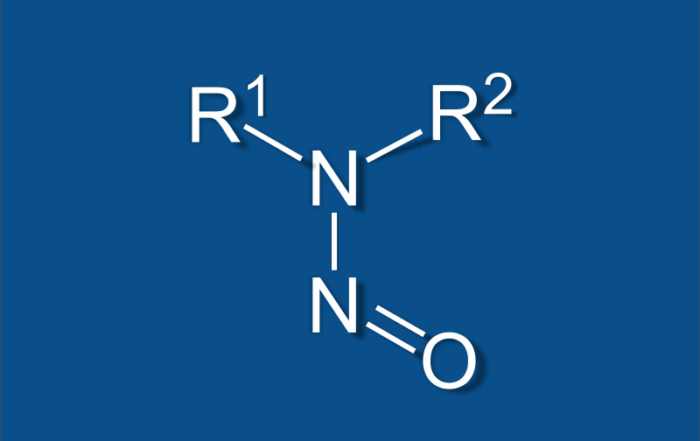
Nitrosamine Impurities Testing With the Updated FDA Guidance
Nitrosamine impurities have recently become a very important topic in the world of pharmaceuticals. Ever since [...]
Nucro-Technics’ COVID-19 Pandemic Response
The COVID-19 pandemic is serious and affects us all. Nucro-Technics is a business that has been deemed [...]
Nitrosamine Testing of Impurities
Health Canada recently sent a letter to Market Authorization Holders (MAHs) about nitrosamine testing of impurities [...]
NDMA Testing in Ranitidine / Zantac
Over the past week or so, drugs containing Ranitidine have been in the news with provocative [...]
DiSARM FEAR with Eurostars: Developing a Treatment for PTSD
Nucro-Technics is very pleased to announce that it has been approved by the Eurostars program as [...]
SCC GLP Certificate of Recognition Update
From March 6-8 of 2019, Nucro-Technics was audited by the Standards Council of Canada (SCC) to [...]